Impact of Anemia in Myelodysplastic Syndromes (MDS)

Up to 90% of patients with MDS develop anemia, and about half of those have Hb < 10 g/dLZeidan AM et al. Blood Rev. 2019;34:1-15. Sekeres MA Taylor J. JAMA. 2022;328:872-880.
Most cases of MDS are idiopathic in nature and patients typically present with non-specific signs and symptoms. Observed cytopenias include anemia, neutropenia, thrombocytopenia, or pancytopenia.Zeidan AM et al. Blood Rev. 2019;34:1-15. Sekeres MA Taylor J. JAMA. 2022;328:872-880. Weinberg OK, Hasserjian RP. Semin Hematol. 2019;56:15-21.
Anemia is an important cause of morbidity and mortality in MDSMalcovati L et al. Haematologica. 2011;96:1433-1440. Platzbecker U et al. Leukemia. 2021;35:2182-2198.
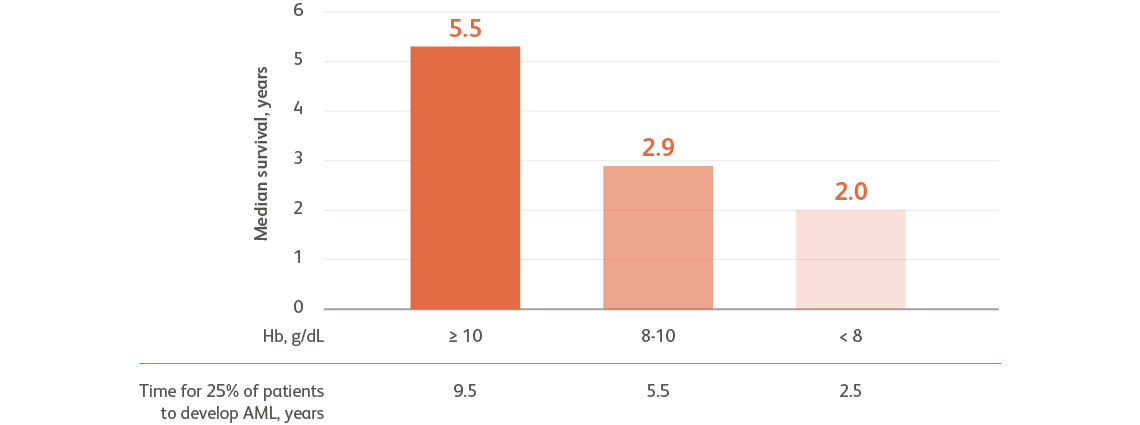
Data are from combined international databases of 7012 patients with primary untreated MDS used to create the IPSS-RGreenberg PL et al. Blood. 2012;120:2454-2465.
AML, acute myeloid leukemia; Hb, hemoglobin, IPSS-R, Revised International Prognostic Scoring System.
- Severe anemia is associated with a higher prognostic risk, a shorter overall survival (OS), and a more rapid evolution to acute myeloid leukemia (AML)Greenberg PL et al. Blood. 2012;120:2454-2465.
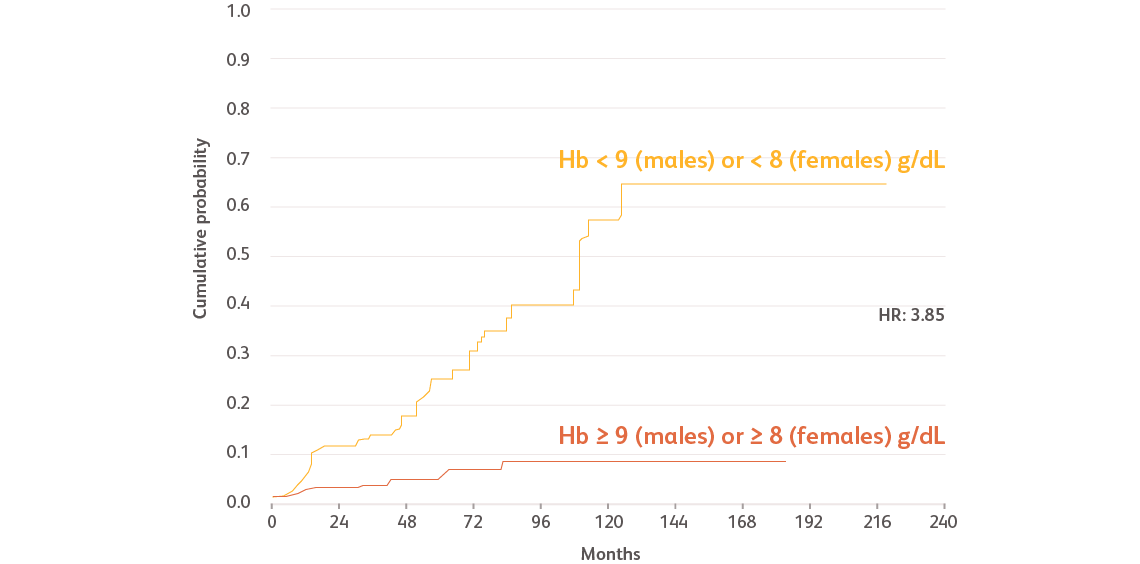
Reproduced with permission from Haematologica.Malcovati L et al. Haematologica. 2011;96:1433-1440.
Data based on a prospective, observational cohort analysis of 840 patients with MDS in Pavia, Italy, and 504 patients with MDS in Düsseldorf, Germany. Cox regression analyses were performed, including age, Hb categories, WHO subgroups, cytogenetic risk groups categorized according to the IPSS, number of cytopenias, and cardiac disease as time-dependent covariates.Malcovati L et al. Haematologica. 2011;96:1433-1440.
Hb, hemoglobin; HR, hazard ratio; IPSS, International Prognostic Scoring System; WHO, World Health Organization.
- Cardiac diseases, including coronary artery disease, congestive heart failure, myocardial infarction, arrhythmia, and heart valve disease, were the most frequently observed comorbidities of anemiaMalcovati L et al. Haematologica. 2011;96:1433-1440.
- Males with Hb levels < 9 g/dL and females with Hb levels < 8 g/dL had significantly more frequent cardiac disease and death compared with those with higher Hb levels (incidence rate ratio [IRR]: 1.70 and 4.22, respectively)Malcovati L et al. Haematologica. 2011;96:1433-1440.
Transfusion dependence negatively impacts the survival of patients with MDSMalcovati L et al. Haematologica. 2006;91:1588-1590. Hiwase DK et al. Am J Hematol. 2017;92:508-514. Braga Lemos M et al. Eur J Haematol. 2021;107:3-23.
 of patients with MDS receive RBC transfusions for anemiaa
of patients with MDS receive RBC transfusions for anemiaa
 of patients receive regular transfusions, with 46.6% considered
of patients receive regular transfusions, with 46.6% considered
as having high transfusion burden (HTB) and 5.8% with low transfusion burden (LTB)b
aData based on a Surveillance, Epidemiology, and End Results (SEER) registry analysis of patients with MDS from 2001 to 2007.Ramsey SD et al. Vox Sang. 2012;102:331-337.
bData based on an observational, retrospective, population-based study using the HemoBase registry, including 292 patients diagnosed with MDS between 2005 and 2017 in the Netherlands. Transfusion burden was defined according to the International Working Group (IWG) 2018 guidelines: non-transfusion dependent (NTD; ≤ 2 U/16 wk), LTB (3-7 U/16 wk), and HTB (≥ 8 U/16 wk).Rozema J et al. Transfusion. 2021;61:2877-2884.
cData based on a practice and treatment survey of 30 European centers.Balducci L. Cancer. 2006;106:2087-2094.
- About half of patients with MDS develop transfusion dependence within 2 years of diagnosis because of its chronicityRozema J et al. Transfusion. 2021;61:2877-2884. Oliva EN et al. Blood Rev. 2021;50:100851. Balducci L. Cancer. 2006;106:2087-2094.
- Some patients with MDS may require more frequent transfusions with disease progressionBalducci L. Cancer. 2006;106:2087-2094.
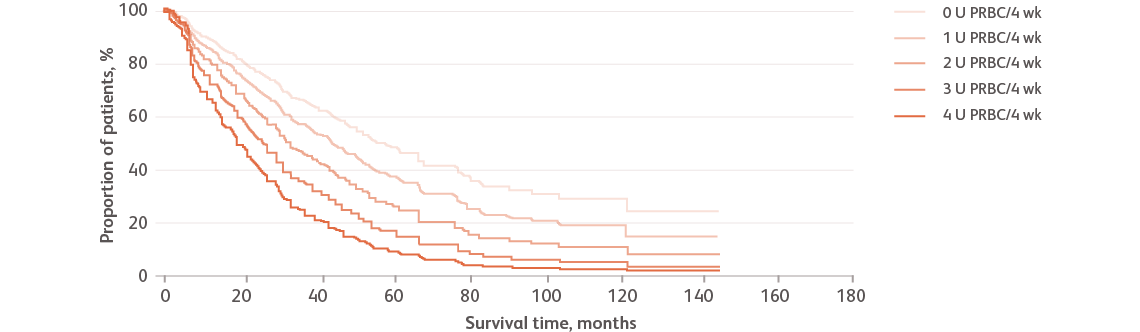
Reproduced with permission from Haematologica.Malcovati L et al. Haematologica. 2006;91:1588-1590.
Data based on a retrospective cohort of 426 Italian patients with MDS studied between 1992 and 2004.Malcovati L et al. Haematologica. 2006;91:1588-1590.
PRBC, packed red blood cells.
- The effect of transfusion dependence is more noticeable in patients with low-risk myelodysplastic syndromes (LR-MDS) and is associated with the severity of transfusion requirements, expressed as the number of packed red blood cells (PRBC) units per month (U/4 wk). An increased transfusion requirement can be an independent predictor of shorter survivalMalcovati L et al. Haematologica. 2006;91:1588-1590.
 transfusion-independent (TI) vs transfusion-dependent (TD)
transfusion-independent (TI) vs transfusion-dependent (TD)
 decreased risk of deathBraga Lemos M et al. Eur J Haematol. 2021;107:3-23.
decreased risk of deathBraga Lemos M et al. Eur J Haematol. 2021;107:3-23.
Hazard ratio (HR): 0.44 (95% Cl: 0.34-0.55)
Data based on a multiple Cox regression analysis in a meta-analysis of 4874 patients with MDS from 11 studies that reported OS outcomes for TI vs TD patients at baseline. Interpretation of these data may be limited because most of the studies in this meta-analysis were from observational, retrospective cohorts and abstracts with response rates and follow-up descriptions missing in large part. Confounders may also be present due to some heterogeneity in study parameters, study design, clinical characteristics, definitions, reliability of results, and risk curves.Braga Lemos M et al. Eur J Haematol. 2021;107:3-23.
- Patients with MDS who are transfusion independent have a 56% decreased risk of death compared with those who require transfusionsBraga Lemos M et al. Eur J Haematol. 2021;107:3-23.
- The HR global effect for OS in the pooled analysis was 0.44 (95% confidence interval [Cl]: 0.34-0.55). Most other clinical outcomes, such as AML progression, non-leukemic death, and progression-free survival (PFS), were also associated or had a trend toward worse prognosis in TD patientsBraga Lemos M et al. Eur J Haematol. 2021;107:3-23.
Frequent transfusions may also cause significant clinical complicationsOliva EN et al. Blood Rev. 2021;50:100851. Balducci L. Cancer. 2006;106:2087-2094.
Transfused patients with MDS have a greater incidence of comorbid conditions compared with non-transfused patientsGoldberg SL et al. J Clin Oncol. 2010;28:2847-2852.

Reproduced with permission from J Clin Oncol.Goldberg SL et al. J Clin Oncol. 2010;28:2847-2852.
Data based on a retrospective review of US Medicare claims of beneficiaries aged ≥ 65 years in 2003 with a 3-year follow-up. A limitation of this study lies in the use of claims data, which are less detailed and have less accurate clinical information. These types of data may also be at risk for over- and/or under-reporting due to coding and naming discrepancies.Goldberg SL et al. J Clin Oncol. 2010;28:2847-2852.
- Among patients receiving transfusions, 82.4% were diagnosed with a cardiac event compared with 67.1% who did not receive transfusions. Diabetes, dyspnea, hepatic disease, and infectious diseases also had a higher prevalence in patients with MDS receiving transfusions during the 3-year follow-upGoldberg SL et al. J Clin Oncol. 2010;28:2847-2852.
Alloimmunization is an immune response against foreign RBC antigens that causes hemolytic reactions, which can make finding compatible blood for future transfusions more difficultThein SL et al. Haematologica. 2020;105:539-544. Molina-Aguilar R et al. Transfus Med Hemother. 2020;47:152-159.

Adapted from Haematologica.Thein SL et al. Haematologica. 2020;105:539-544.
RBC, red blood cell.
- The risk of alloimmunization increases with an increasing number of RBC transfusionsZalpuri S et al. BMJ Open. 2012;2:e001150.
Chronic transfusion may also lead to iron accumulationWeber S et al. Front Immunol. 2020;11:627662.

ROS, reactive oxygen species.
- Excess iron can be toxic to several organs and can cause tissue damageWeber S et al. Front Immunol. 2020;11:627662.
- It can also affect components of the immune system and increase the risk of infectionsWeber S et al. Front Immunol. 2020;11:627662.
Living with MDS places a heavy burden on patients’ daily livesRia R et al. Clin Interv Aging. 2009;4:413-423. Stauder R et al. Leukemia. 2018;32:1380-1392.
- Several studies have shown that the health-related quality of life of patients with MDS is significantly worse compared with the general populationRia R et al. Clin Interv Aging. 2009;4:413-423. Stauder R et al. Leukemia. 2018;32:1380-1392.
- Similarly, caregivers also suffer in a manner similar to patients, reporting a high degree of distress and below average emotional, social, and functional wellbeingDiNardo KW et al. Blood. 2022;140:8122-8123

Physical problems
- MDS causes a substantial and persistent functional decrement in a variety of areas, partially due to fatigueRia R et al. Clin Interv Aging. 2009;4:413-423.
- 41% of patients with MDS reported moderate or severe mobility issuesStauder R et al. Leukemia. 2018;32:1380-1392.
Data from a study of 1985 newly diagnosed patients in the European Myelodysplastic Syndromes Registry (EUMDS) registry with IPSS low or intermediate-1 MDS compared with the general population in Europe matched on age and sex.Stauder R et al. Leukemia. 2018;32:1380-1392.

Emotional problems
- Patients have often viewed the emotional impact of MDS as being more problematic than the physical consequencesRia R et al. Clin Interv Aging. 2009;4:413-423.
- 37.9% of patients with MDS reported moderate or severe issues with anxiety/depressionStauder R et al. Leukemia. 2018;32:1380-1392.
Data from a study of 1985 newly diagnosed patients in the EUMDS registry with IPSS low or intermediate-1 MDS compared with the general population in Europe matched on age and sex.Stauder R et al. Leukemia. 2018;32:1380-1392.

Role functioning
- The disease may affect employment, necessitating time off from work or otherwise compromising career opportunitiesSoper J et al. Patient Relat Outcome Meas. 2022;13:31-38.
- 36.1% of patients with MDS reported moderate or severe problems with usual activities (eg, work, housework, or leisure activities)Stauder R et al. Leukemia. 2018;32:1380-1392.
Data from a study of 1985 newly diagnosed patients in the EUMDS registry with IPSS low or intermediate-1 MDS compared with the general population in Europe matched on age and sex.Stauder R et al. Leukemia. 2018;32:1380-1392.

Social functioning
- Transfusion requirements may disrupt routines and take time and attention away from family and friendsSoper J et al. Patient Relat Outcome Meas. 2022;13:31-38.
- 34% of patients receiving blood transfusions felt their transfusions were a burden to familySekeres MA et al. Oncologist. 2011;16:904-911.
Data from an internet-based survey of 358 patients with MDS, of whom 234 were receiving transfusions.Sekeres MA et al. Oncologist. 2011;16:904-911.